jinkō
A complete solution for trial simulation
& design optimization
A platform to simulate trials and foster collaboration
« jinkō helped my team explore the best treatment combinations for our PhII clinical trial, with an unlimited number of trial designs at limited cost. »
« I use jinkō to build models for our clinical team. My non-modeler colleagues can then run their in silico clinical trials with full autonomy, which frees my time to cover more projects in our pipeline. »
« Thanks to jinkō we can provide the rationale for dose selection, and thereby meet regulators’ expectations. »
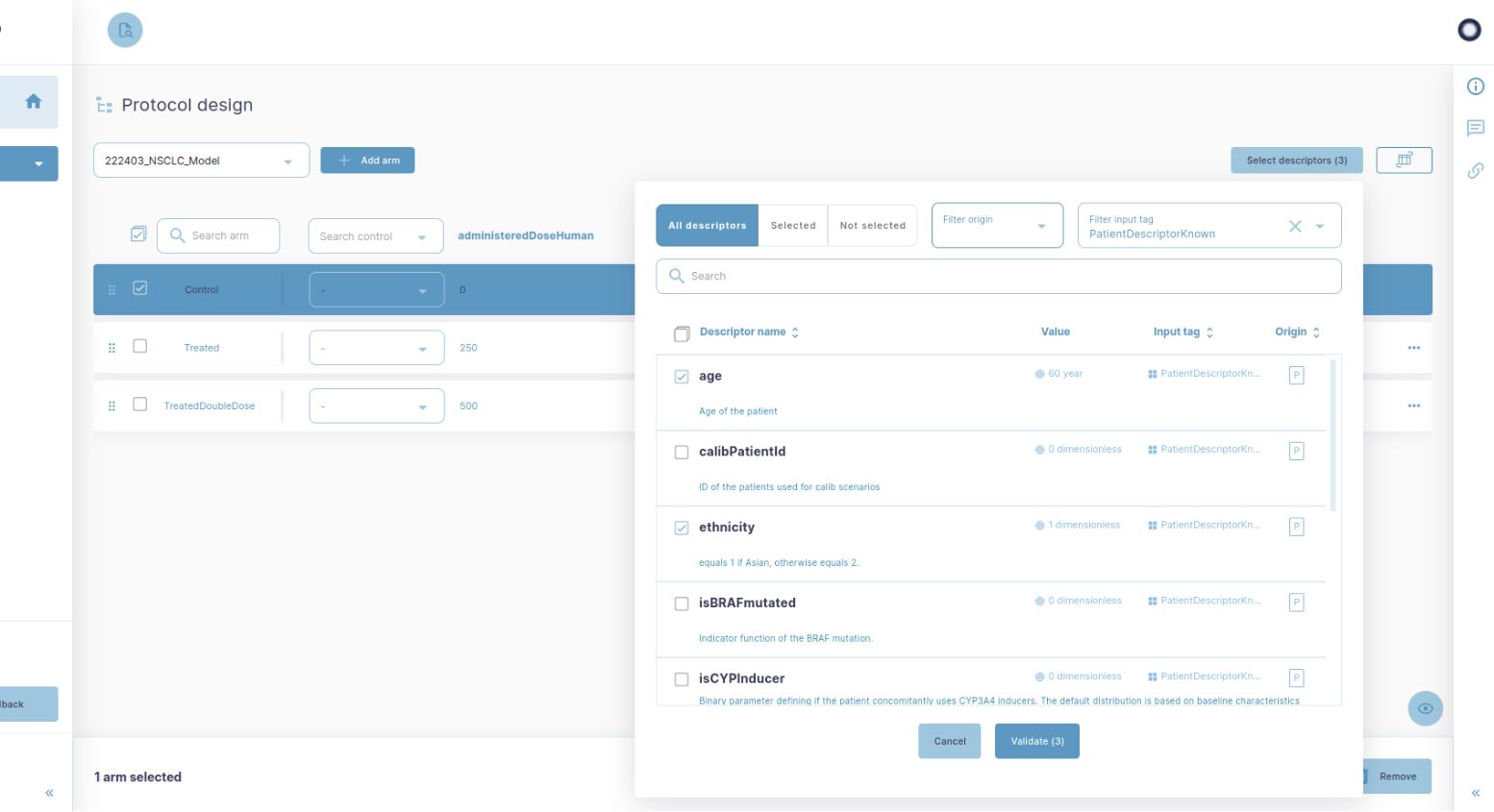
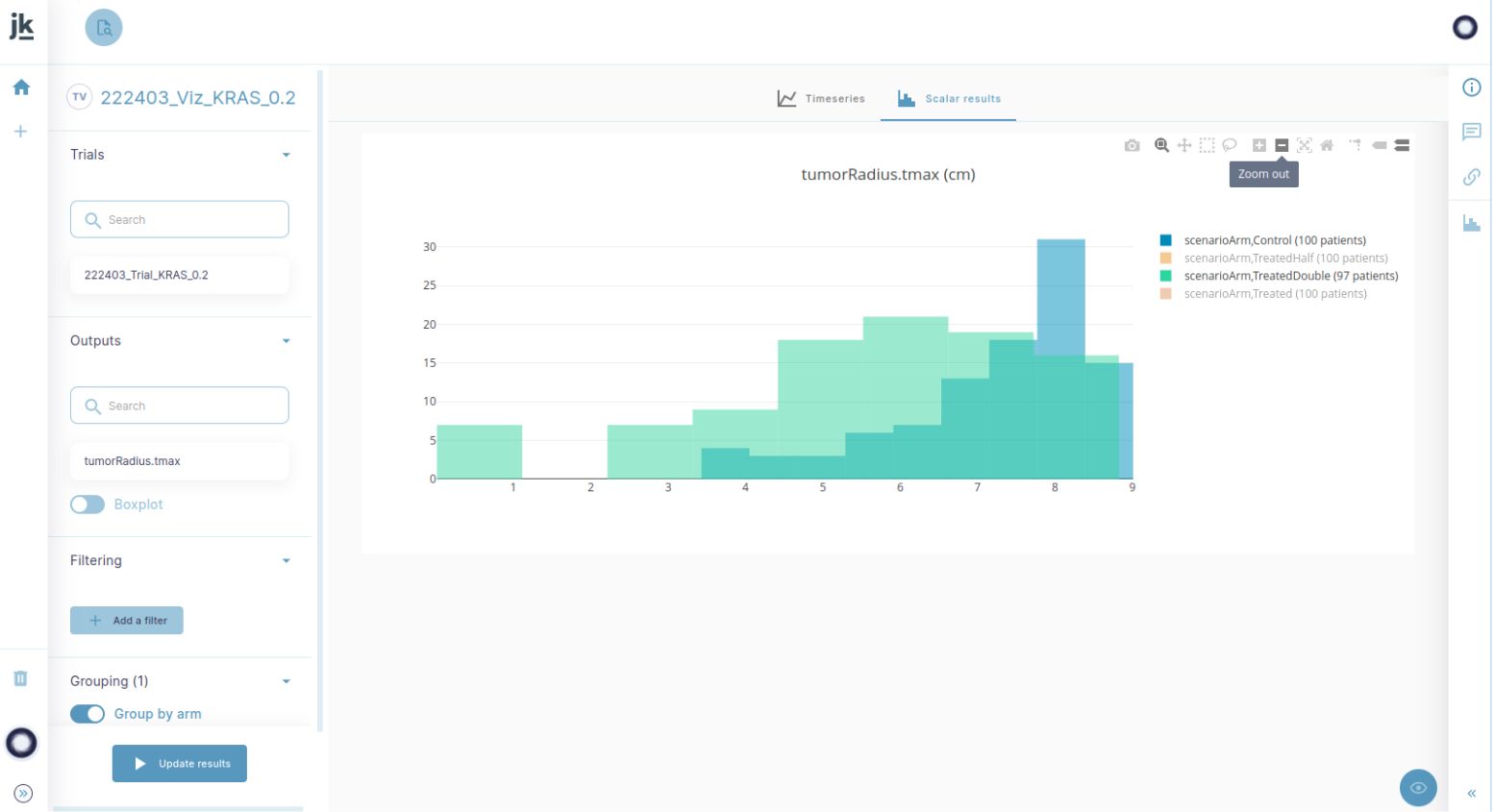
« We provide clinicians with clear visualisations of the impact of a given variable on the study outcome. This is particularly useful to refine inclusion and exclusion criteria. »
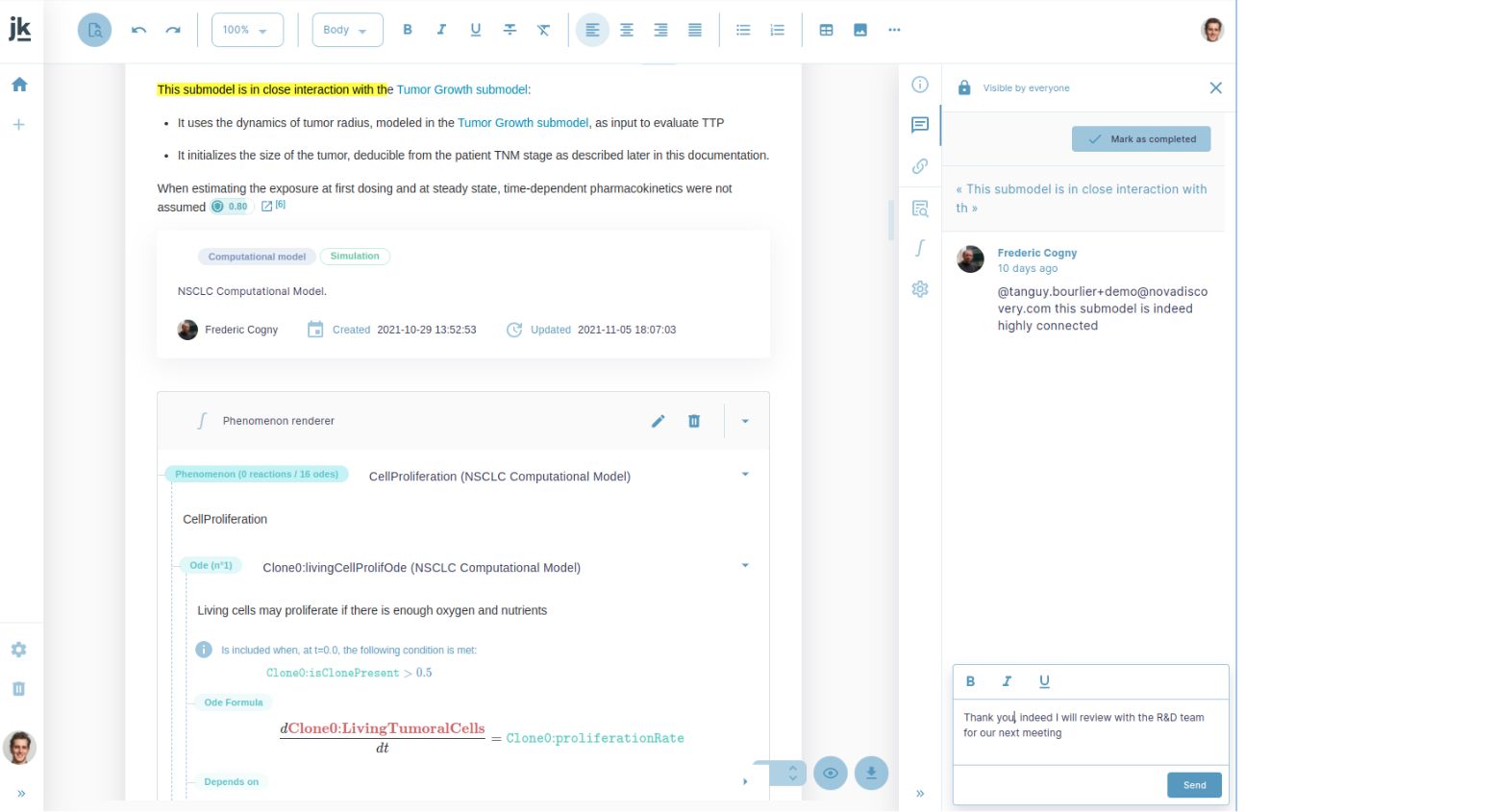
« I enjoy jinkō’s collaborative, transparent approach to literature review. Everything is traceable, meaning I can always easily go back to the primary knowledge source and therefore have full confidence in what has gone into the disease model. »
« I can generate the exact clinical data required by each HTA body to feed my HEOR model. »
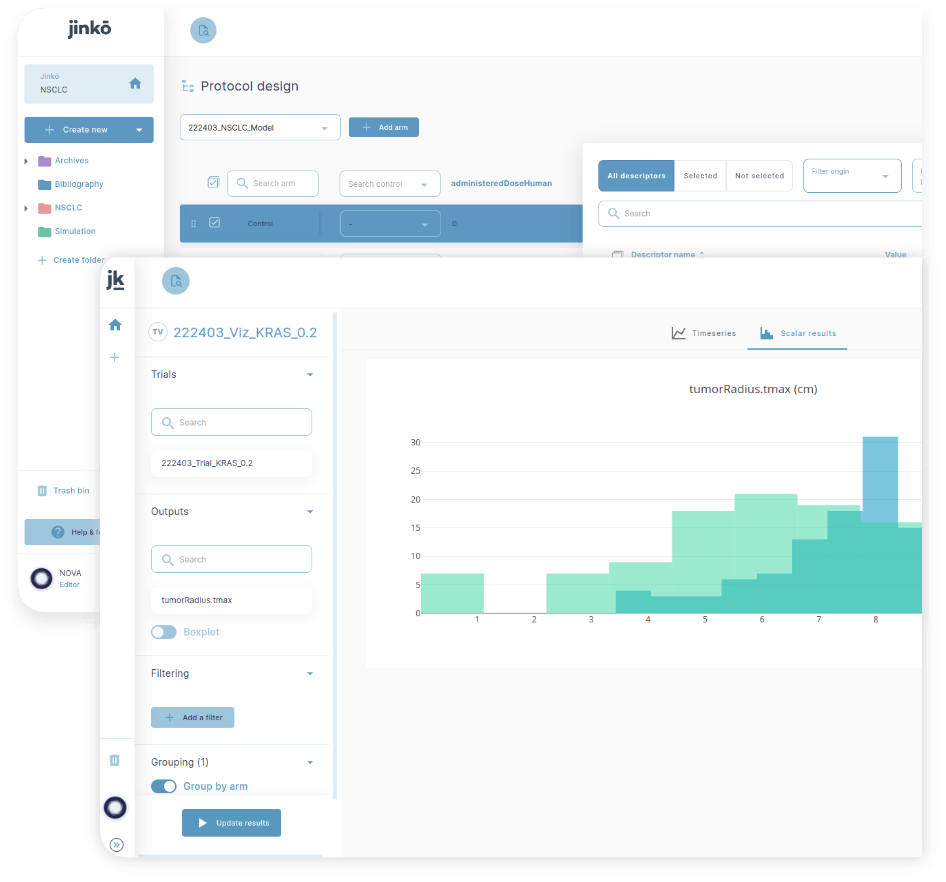
Features jinkō platform
Go further
about in silico clinical trials ?